kidney failure seizure dog
What Are the Symptoms of a Dog Dying From Kidney Failure?

If your dog has been diagnosed with this renal disease or you're worried they're showing signs associated with end-stage kidney failure, a lot is probably going through your mind right now. At the forefront might be which stage of kidney disease does your dog fall under, and how can you make them as comfortable as possible? It's advisable to educate yourself about what to expect as your pet's illness progresses. That way, you can give them the best quality of life all the way through their final days.
Dog Kidney Failure Stages
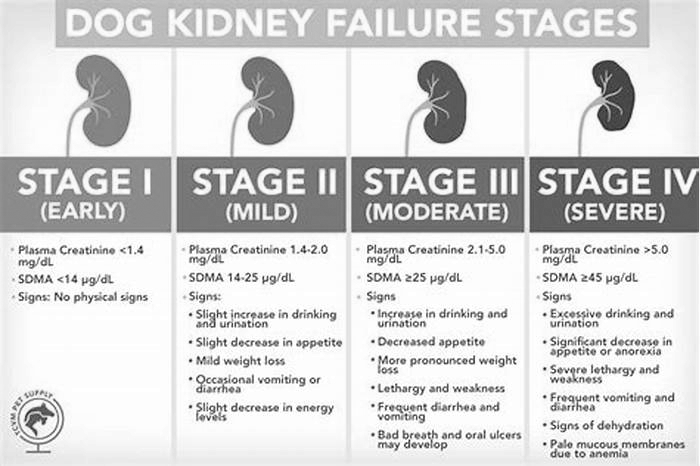
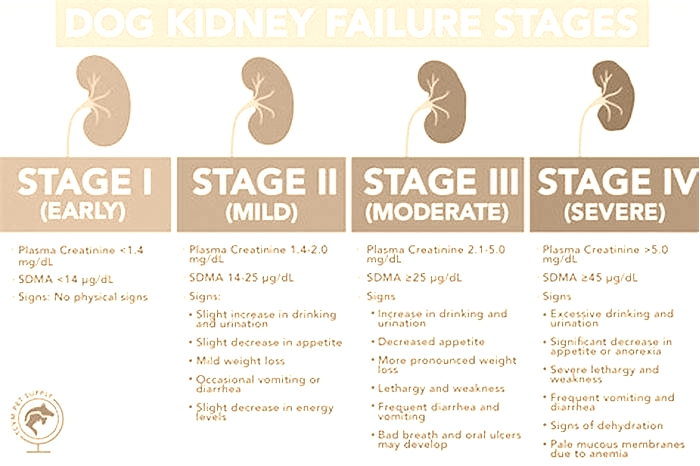
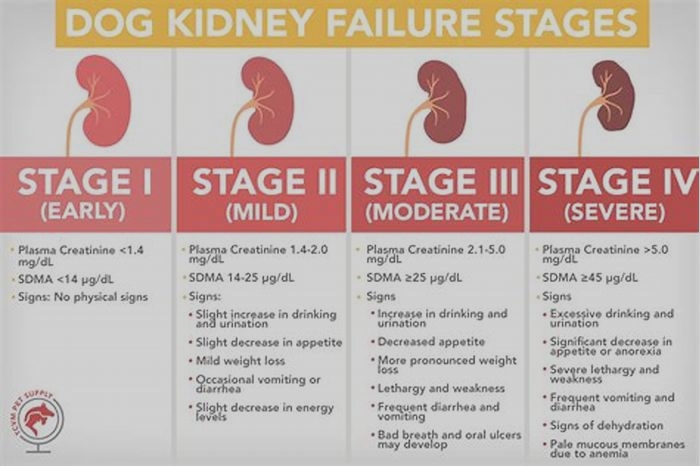
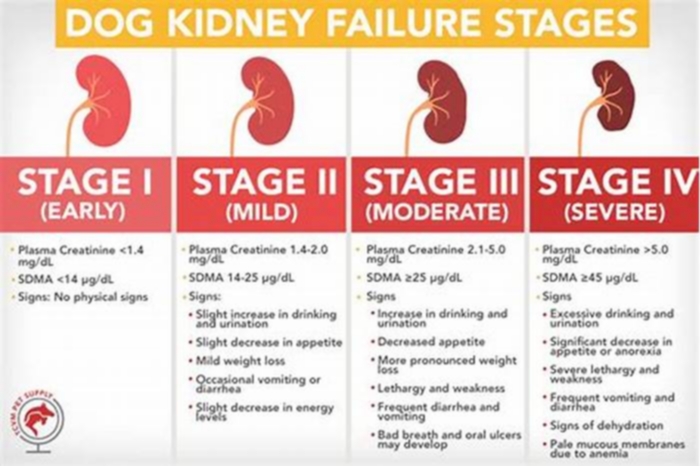
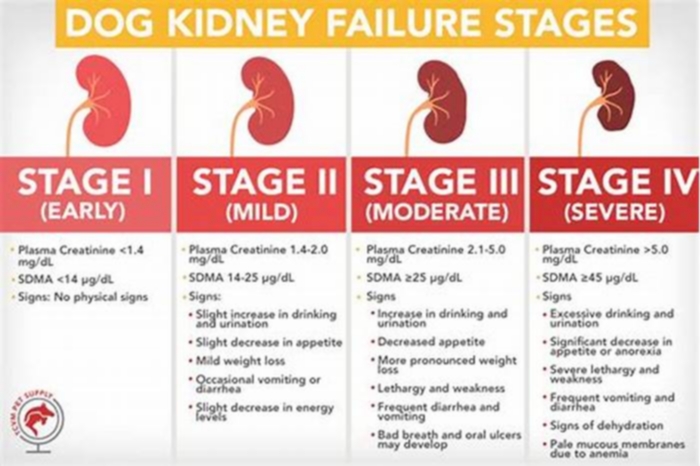
Dogs with kidney failure go through a series of four stages, from diagnosis through the eventual death of the animal. These stages do not necessarily occur within rapid succession. A dog can go through them over the course of a few months or even years. Veterinarians determine the stage your dog is in by testing the urine to look for signs of the deterioration of the kidney's functions and the blood for symmetric dimethylarginine (SDMA) levels.
The Four Stages of Kidney Failure Chart
The canine kidney failure stages are determined by the levels of creatinine and SDMA, as well as the urine-to-protein (UPC) ratio and the animal's systolic blood pressure.
| Dog Kidney Failure Stage | Creatinine (in mg/dL) | SDMA (in g/dL) |
| Stage 1 | less than 1.4 | less than 18 |
| Stage 2 | 1.4 to 2.0 | 18 to 35 |
| Stage 3 | 2.1 to 5.0 | 36 to 54 |
| Stage 4 | greater than 5.0 | greater than 54 |
Other clinical indications that a dog is in the stages of kidney failure are:
- UPC Ratio:
- Nonproteinuric: less than 0.2
- Borderline proteinuric: 0.2 to 0.5
- Proteinuric: greater than 0.5
- Systolic blood pressure (in mmHg):
- Normotensive: less than 150
- Borderline hypertensive: 150 to 159
- Hypertensive: 160 to 179
- Severely hypertensive: greater than 180
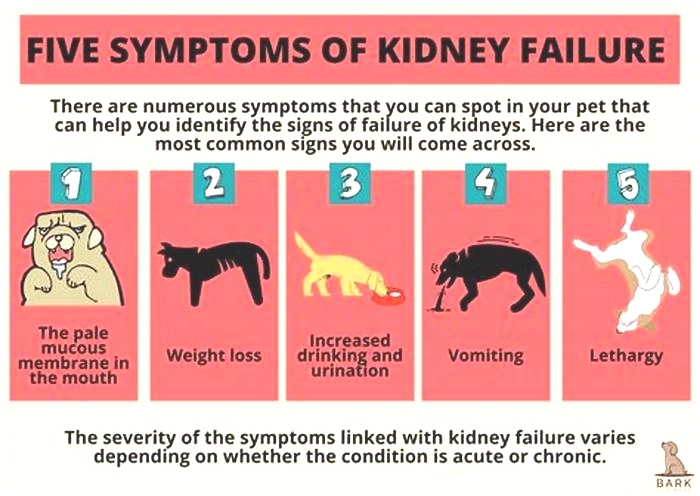
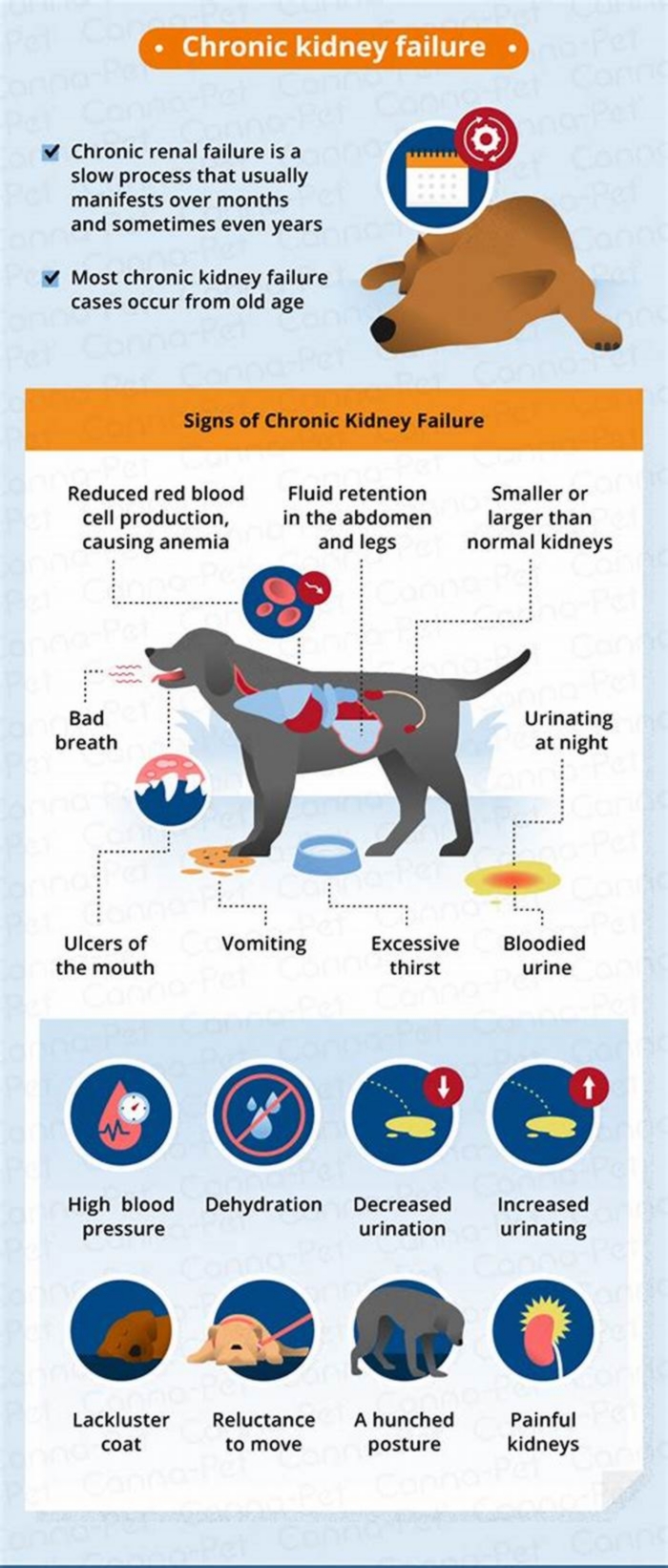
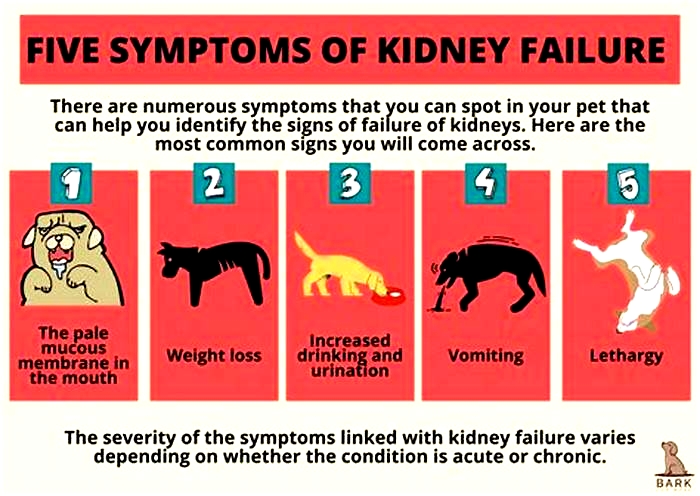
Symptoms of End-Stage Kidney Failure in Dogs
The most common signs a dog is dying from kidney failure include:
- Uremia: The buildup of waste products in the body produces a distinctive ammonia smell that is especially apparent on the breath.
- Pale, dry gums: The gums are duller and dry to the touch.
- Mouth ulcers: Uremia causes raw mouth ulcers that are painful.
- Bloodshot eyes: The whites of the eyes are bloodshot.
- Increased thirst: An affected dog drinks water excessively.
- Increased urination: The dog will urine large volumes of dilute urine.
- Dehydration: Despite more fluid intake, the dog is dehydrated.
- Decreased appetite: The dog loses interest in food.
- Weight loss: The dog steadily loses weight.
- Gradual loss of fat and muscle mass: The weight loss affects both fat and muscle mass and can cause emaciation.
- Dull coat that sheds excessively: The lackluster coat constantly sheds and looks unkempt.
- Lethargy: The dog has little energy or interest in moving around.
- Fatigue: The dog sleeps most of the day and night with only brief periods of wakefulness.
- Vomiting: The dog vomits frequently and cannot keep food down.
- Anemia: The dog may develop anemia.
- High blood pressure: The dog has elevated blood pressure.
- Incontinence: A dog cannot control their urination.
- Difficulty breathing: The dog has problems breathing normally.
- Slowing heart rate: A faster heart rate is generally present with kidney failure, but the heart rate begins to slow down during the end stage of the disease.
- Depression: The dog seems sad and does not respond to any of their favorite things.
- Low temperature: Dogs in their last days of kidney failure can experience hypothermia or a low body temperature.
- Lack of interest in surroundings: The dog is unaware of or disinterested in their surroundings.
- Disorientation: The dog acts confused at times.
- Loss of balance and coordination: The dog appears clumsy and unsteady on their feet.
- Trembling or shaking: The dog has tremors or episodes of shaking.
- Seizures: The dog suffers periodic seizures, one of the major signs of end-stage kidney failure.
Keeping Your Pet Comfortable
Watching your pet go through this can be very difficult. However, there are things you can do to help keep your dog comfortable during the final stages of kidney disease.
- Spend as much time as possible with your dog. Even being in the same room will be soothing to them.
- Make sure your dog's resting area is quiet, warm, and cozy. Provide them with their favorite blanket and toy.
- Protect your pet from other pets or people who may be too rough with them. Supervise interactions with children and teach them to be gentle with the dog.
- Pet your dog and talk to them frequently.
- Change your dog's bedding often and keep them clean and dry. Brush their fur for dry cleaning. Clean their fur with a sponge bath solution of hypoallergenic pet shampoo.
- Feed your pet a low-protein dog food appropriate for a kidney failure diet.
- If your dog refuses to eat or has trouble eating, ask the veterinarian about other feeding options such as an esophagostomy tube to keep them nourished.
- Monitor your dog's temperature and keep them warm with plenty of cozy blankets.

Last Days of a Dog Dying From Kidney Failure
While a dog owner may fear that entering the final stage of kidney failure means their dog's passing away is imminent, it is difficult to predict how long the does has left. In general, you can expect your dog to pass away within three months of moving into stage 4, though some dogs may thrive for up to a year.
It depends on the associated symptoms and other conditions that may arise due to the dog's poor health. Your dog's age is another factor. There's a lot to keep in mind, a lot to hope for, and the reality that you're facing the end of your dog's life.
Fast Fact
Controlling your dog's diet can help during their struggle with renal failure. Carefully discuss nutrition with your dog's veterinarian.
When to Consider Euthanization
When a dog enters end-stage renal failure, your veterinarian may recommend an end-of-life home treatment plan or a hospice program to make your pet's last days comfortable and maintain your pet's quality of life. For end-stage kidney failure, a treatment plan may include dialysis, a stomach tube, intravenous therapy, pain medication, and methods to care for an incontinent pet.
Depending on their symptoms, your dog may not necessarily be in severe pain, but they will be uncomfortable at the least from other symptoms, including frequent vomiting and diarrhea, lethargy and depression, and constant dehydration. Your veterinarian may recommend euthanasia if a dog is suffering, unresponsive to pain management, or too weak to handle necessary life-sustaining treatment.
Dealing With the Loss
It's hard to come to terms with the fact that a pet is dying. Find comfort in the fact that your dog appreciates your loving care in their final days. They know you love them and take comfort in your presence and all that you do to make their life easier.
2024 LoveToKnow Media. All rights reserved.
Use of Levetiracetam in Epileptic Dogs with Chronic Kidney Disease: A Retrospective Study
1. Introduction
Epilepsy, which affects 50 million people around the world, is the most common neurological disorder [1]. In veterinary medicine, epilepsy is also the most common neurological disorder [2]. The key treatment for this condition is antiepileptic drugs (AEDs), of which the most commonly used are phenobarbital and potassium bromide [3]. However, seizures are not well controlled in 2030% of dogs with epilepsy [4]. Additionally, dogs can experience severe adverse effects with conventional AED treatment [4]. For these patients, the assessment of new AEDs for the management of epilepsy is essential [5].
Levetiracetam (LEV) is a structurally novel AED that was approved for use in humans as an adjuvant drug for partial-onset seizures in 1999 [6]. LEV is not metabolized via the hepatic cytochrome P450 system [7]. Because of its minimal hepatic metabolism, LEV is favored for use in geriatric or critical patients [7,8,9]. Furthermore, in human medicine, LEV has a minimal effect on the distribution of other AEDs because of its unique metabolic pathway [7,8,9]. Therefore, LEV is particularly favored when initiating polytherapy [10]. Accordingly, LEV has particular uses in special populations [7,8,9]. Based on its encouraging results in treating human epilepsy, levetiracetam is being used frequently in veterinary medicine, especially in dogs [11]. However, there are scant reports of its use in special populations of veterinary patients.
Compared to other AEDs, LEV has a broad margin of safety [7,8]. However, in human medicine, some patients experience undesirable side-effects and toxicity, particularly in special populations, including renal, hepatic, or cardiac insufficiency [12,13]. The usual drug dosage is established for individuals with normal renal function and metabolism [13]. In patients with impaired renal and/or hepatic function, the elimination of drugs may be markedly reduced [9,13]. In such patients, the conventional dosage regimen causes drug accumulation [13,14]. Therefore, in special populations, identifying pharmacokinetic alterations is important to guide prescription strategies [12].
The half-life of LEV elimination is extended to 1011 h in elderly human patients, and this is also extended in patients with renal disease [12,13]. Therefore, LEV dosing is adjusted in patients with impaired renal function according to the status of the patients renal function [12]. Numerous studies in human medicine report individualized LEV dosing according to the status of the patients renal function [8,9,12,13]. However, there are not reports concerning individualized LEV dosing according to the status of the patients renal status in veterinary medicine.
Evaluating the use of LEV in preexisting CKD dogs was the goal of this retrospective study, resulting in the need for dose adjustment in patients with kidney disease.
2. Materials and Methods
2.1. Study Population
For this retrospective study, the patient databases of the Seoul National University Veterinary Medical Teaching Hospital (SNU VMTH) were searched for cases where LEV had been prescribed. The study period was between 5 January 2011 and 28 December 2019. In total, 138 dogs received LEV over a period of 9 years. Hospital records for all eligible patients were reviewed. Information and data necessary for patient evaluation were obtained from the medical records using an electronic chart program (E-friends; pnV, Jeonju, Korea).
2.2. Inclusion Criteria
The patient inclusion criteria of this study were as follows: (1) dogs with the presence of seizure or epilepsy and who were treated with oral LEV; (2) who had been diagnosed with or without CKD before LEV administration; (3) and were administered oral LEV for 7 days.
2.3. Exclusion Criteria
The patient exclusion criteria of this study were as follows: (1) patients who had not been screened for kidney impairments before LEV administration; (2) who were lost to follow up; (3) or who were missing serum blood urea nitrogen (BUN), creatinine, or phosphorus (P) data.
2.4. Blood Test Results Analysis
At least 12 h after fasting, blood samples were taken. The components of the blood tests were as follows: BUN, creatinine, P, and symmetric dimethylarginine (SDMA) levels. To determine the effects of LEV on patients kidney condition, we compared the blood test results before and after LEV treatment. The LEV-pretreatment blood test results were collected within 1 month prior to starting LEV administration. The LEV post-treatment results were obtained between 2 and 64 days after starting oral LEV administration according to patients condition and revisit interval.
Serum BUN was evaluated using standard definitions for clinically significant differences from baseline [15,16]. In patients with normal or low baseline BUN values, a relevant increase in serum BUN was defined as a 25% increase over the upper normal limit, and for patients with initially greater than normal BUN values, a 25% increase higher than baseline was considered [15,16]. Serum creatinine and p values were evaluated following the same method as the one used for serum BUN evaluation.
2.5. CKD Evaluation Using the IRIS CKD Diagnosis Guidelines
We investigated whether CKD, defined by the International Renal Interest Society (IRIS) CKD diagnostic guidelines, had been diagnosed before starting LEV administration. CKD staging (CKD stages 14) was determined according to the IRIS guidelines for staging CKD [17]. All available information, including serial serum creatinine concentrations, urinalysis, and diagnostic imaging findings, were used for the diagnosis of CKD and for the determination of the CKD stage, before starting LEV administration [17].
2.6. Epilepsy Frequency and Days Prior to and during LEV Treatment
The data collected from the patient records included age and weight at the start of LEV treatment, the total number and days of epilepsy prior to starting LEV administration, and the total number and days of epilepsy occurring during LEV treatment. The total number of epilepsy incidents during LEV treatment that occurred within 1 month after starting LEV administration was counted [18]. Additionally, the total number of days on which epilepsy occurred during the 1 month after starting LEV treatment was counted [18].
2.7. Levetiracetam Administration
All of the patients in this study were given a daily maintenance dose of LEV (Keppra; UCB Pharma SA, Belgium; Levetiracetam; Rhino Bio, Daejeon, Korea) per os (PO), depending on the patients status, and side-effects and therapeutic responses were monitored. LEV was administered at an initial dose of 10.045.0 mg/kg PO, q812h. The LEV dose was adjusted based on clinical response. LEV was used as the primary or as an add-on therapy, depending on the patients conditions. When used as add-on therapy, LEV was used in combination with phenobarbital, potassium bromide (KBr), zonisamide, or diazepam.
Side-effects of oral LEV were recorded; in particular, whether the following variable symptoms were more frequently provoked during LEV treatment was monitored: ataxia, sedation, decreased appetite, polydipsia, vomiting, diarrhea, hypersalivation, and behavior changes (showing aggression) [10,11,18]. The side-effects that occurred during LEV treatment were monitored for 1 month after the start of LEV.
2.8. Statistical Analysis
Statistical analysis was performed using commercial software (R package, ver. 3.1.1; The R Foundation for Statistical Computing, Vienna, Austria). Analytical data are presented as mean standard deviation (SD). Data are presented as median with range and SD. Differences between the variables from the CKD group and the non-CKD group were tested using Fishers exact test or the chi-square test. p-values < 0.05 were regarded significant.
3. Results
3.1. Study Animals
In total, 138 dogs with seizure or epilepsy were given oral LEV over a period of 9 years, of which 62 patients met the inclusion criteria. A further 25 patients were excluded based on the exclusion criteria. Finally, 37 dogs were included in this study. We divided these patients into a CKD group (n = 20) and a non-CKD group (n = 17).
The CKD group included 20 dogs with a mean age of 12.53 3.78 years (range, 2.5817.5 years) and a median weight of 5.03 2.78 kg (range, 1.813.1 kg) when treatment with LEV began (). Several breeds were included, with the Maltese being the most common (20%) (). Dogs included both intact and neutered females and males (). The CKD groups etiological diagnoses are presented in .
Table 1
Characteristics of patients with the CKD group/non-CKD group in the present study.
| CKD Group (n = 20) | Non-CKD Group (n = 17) | |
|---|---|---|
| Variables | No. of Dogs (%) | No. of Dogs (%) |
| CKD stage before LEV administration | ||
| non-CKD | 0 (0%) | 17 (100%) |
| CKD stage 1 | 12 (60%) | 0 (0%) |
| CKD stage 2 | 8 (40%) | 0 (0%) |
| CKD stage 3 or 4 | 0 (0%) | 0 (0%) |
| Breeds | ||
| Maltese | 4 (20%) | 7 (41.18%) |
| Yorkshire Terrier | 2 (10%) | 2 (11.76%) |
| Pekingese | 2 (10%) | 0 (0%) |
| Cocker Spaniel | 2 (10%) | 2 (11.76%) |
| Poodle | 2 (10%) | 0 (0%) |
| Mixed breed | 2 (10%) | 3 (17.65%) |
| Chihuahua | 0 (0%) | 2 (11.76%) |
| Afghan Hound | 0 (0%) | 1 (5.88%) |
| Sex | ||
| Male | 3 (15%) | 4 (23.53%) |
| Male castrated | 3 (15%) | 4 (23.53%) |
| Female | 4 (20%) | 1 (5.88%) |
| Female spayed | 10 (50%) | 8 (47.06%) |
| Body weight at start of LEV, kg | ||
| Mean SD (range) | 5.03 2.78 kg(range, 1.813.1 kg) | 5.44 5.56 kg(range, 1.2525.5 kg) |
| Age at start of LEV, years (minmax years) | ||
| Mean SD (range) | 12.53 3.78 years(range, 2.5817.5 years) | 10.26 3.73 years(range, 2.0817.25 years) |
Table 2
LEV-based therapy in dogs of the CKD group/non-CKD group.
| CKD Group (n = 20) | NonCKD Group (n = 17) | |
|---|---|---|
| Variables | No. of Dogs (%) | No. of Dogs (%) |
| Indications of LEV | ||
| Undiagnosed epilepsy | 11 (55%) | 6 (35.30%) |
| Idiopathic epilepsy | 3 (15%) | 3 (17.65%) |
| Hydrocephalus | 1 (5%) | 3 (17.65%) |
| Hydrocephalus with syringohydromyelia | 2 (10%) | 1 (5.88%) |
| Chiari-like malformation | 0 (0%) | 2 (11.76%) |
| Meningoencephalitis of unknown etiology | 1 (5%) | 0 (0%) |
| Hydrocephalus with meningoencephalitis of unknown etiology | 0 (0%) | 1 (5.88%) |
| Geriatric vestibular disease suspected | 1 (5%) | 0 (0%) |
| Reactive epilepsy | 1 (5%) | 0 (0%) |
| Brain tumor | 0 (0%) | 1 (5.88%) |
| Variables | Value | Value |
| Initial dose of LEV administered | ||
| Mean SD (range) | 21.94 8.36 mg/kg (range, 10.045.0 mg/kg) | 20.58 8.72 mg/kg (range 10.030.0 mg/kg) |
| Duration of oral LEV therapy (days) | ||
| Mean SD (range) | 147.85 183.54 days (range 8613 days) | 399.82 761.05 days (range 22649 days) |
The non-CKD group consisted of 17 dogs with a mean age of 10.26 3.73 years (range, 2.0817.25 years) and a median weight of 5.44 5.56 kg (range, 1.2525.5 kg) when LEV treatment began (). Several breeds were recorded, with Maltese once again being the most frequent (), and both intact and neutered males and females were included (). The non-CKD groups etiological diagnoses are also presented in .
3.2. Pre-Existing CKD Evaluation Based on IRIS CKD Diagnostic Guidelines
In the CKD group, most patients (60%) were CKD stage 1, while the remainder were CKD stage 2; there were no CKD stage 3 or 4 patients ().
3.3. LEV-Based Therapy in Both Groups
All of the patients in this study were given LEV dosages of 10.045.0 mg/kg orally, q812h. Most patients (n = 33, 89.19%) did not receive a loading dose. The mean duration of the oral LEV therapy in this study was 263.62 547.81 days (range, 22649 days days).
In the CKD group, the initial LEV dose was 21.94 8.36 mg/kg (range, 10.045.0 mg/kg) PO, q812h. In the non-CKD group, this dose was 20.58 8.72 mg/kg (range 10.030.0 mg/kg) PO, q812h (). The mean duration of the oral LEV therapy in the CKD group was 147.85 183.54 days (range, 8613 days), and in the non-CKD group it was 399.82 761.05 days (range, 22649 days) ().
Of the 20 CKD patients, 10 patients (50%) received LVE monotherapy, while the others received LEV in combination with phenobarbital, KBr, zonisamide, and diazepam. Four patients (20%) received LEV in combination with phenobarbital, two patients (10%) received LEV in combination with zonisamide, one patient (5%) received LEV in combination with diazepam, one patient (5%) received LEV in combination with phenobarbital and zonisamide, one patient (5%) received LEV in combination with phenobarbital and potassium bromide, and one patient (5%) used all 5 drugs simultaneously.
Of the 17 non-CKD patients, 9 patients (52.94%) received LEV monotherapy. Two patients (11.76%) received LEV in combination with phenobarbital, one patient (5.88%) received LEV in combination with zonisamide, one patient (5.88%) received LEV in combination with phenobarbital and zonisamide, one patient (5.88%) received LEV in combination with phenobarbital and diazepam, two patients (11.76%) received LEV in combination with zonisamide and gabapentin, and one patient (5.88%) received LEV in combination with phenobarbital, KBr, and zonisamide simultaneously. No drug interactions with LEV were identified among the study patients.
3.4. Outcomes of Patients on LEV Therapy
In the CKD group, the mean number of seizures prior to starting LEV was 59.7 161.7 (range, 0735), and the mean number of seizure days before LEV was 17.55 30.96 (range, 0122) (). In the non-CKD group, the mean number of seizures prior to starting LEV was 42.59 71.313 (range, 1275), and the mean number of seizure days prior to LEV treatment was 16.65 25.72 (range, 189) (). The total number of seizures and the total days of seizures during LEV treatment during the 1 month after the start of LEV treatment was 13.55 38.89 (range, 0162) and 2.8 7.05 (range, 030), respectively, in the CKD group (). In the non-CKD group, during LEV treatment, the total mean number of seizures while on LEV treatment was 7.18 12.60 (range, 048), and the total mean number of seizure days while on LEV treatment was 1.59 1.94 (range, 06) ().
Table 3
Seizure frequency of the CKD group/nonCKD group.
| CKD Group (n = 20) | NonCKD Group (n = 17) | |
|---|---|---|
| Variables | Value | Value |
| Epilepsy frequency prior to LEV treatment | ||
| Mean SD (range) | 59.7 161.70(range, 1735) | 42.59 71.31(range, 1275) |
| Days of epilepsy prior to LEV treatment | ||
| Mean SD (range) | 17.55 30.96(range, 1122) | 16.65 25.72(range, 189) |
| Epilepsy frequency during LEV treatment | ||
| Mean SD (range) | 13.55 38.88(range, 0162) | 7.18 12.60(range, 048) |
| Days of epilepsy during LEV treatment. | ||
| Mean SD (range) | 2.8 7.05(range, 028) | 1.59 1.94(range, 06) |
In general, the side-effects of LEV include ataxia, sedation, anorexia, polydipsia, vomiting, diarrhea, hypersalivation, and behavior changes (showing aggression). Of the 37 patients, 26 patients (70.27%) showed adverse effects during LEV administration. Most patients showed more than one side-effect at the same time (). More dogs in the CKD group were reported to have adverse effects than in the non-CKD group, and there was a statistically difference between the two groups (85% vs. 52.94%, p = 0.033). In the CKD group, a significant increase in sedation was identified during LEV treatment compared to baseline, but it was not statistically significant compared to the non-CKD group (55 versus 41.18%, p = 0.40). No dogs showed life-threatening adverse effects.
Table 4
Side-effects of the oral LEV were observed in the CKD group/nonCKD group.
| CKD Group(n = 20) | NonCKD Group(n = 17) | ||
|---|---|---|---|
| Variables | No. of Dogs (%) | No. of Dogs (%) | p-Value |
| Any adverse event | 17 (85%) | 9 (52.94%) | 0.033 |
| Sedation | 11 (55%) | 7 (41.18%) | 0.40 |
| Ataxia | 5 (25%) | 3 (17.65%) | NA |
| Anorexia | 9 (45%) | 2 (11.76%) | NA |
| Polydipsia | 2 (10%) | 0 (0%) | NA |
| Vomiting | 7 (35%) | 1 (5.88%) | NA |
| Diarrhea | 3 (15%) | 2 (11.76%) | NA |
| Hypersalivation | 3 (15%) | 0 (0%) | NA |
| Behavior changes (showing aggression) | 1 (5%) | 0 (0%) | NA |
3.5. Clinically Relevant Increases in Serum BUN, Creatinine, and Phosphorus after LEV Administration
When all of the eligible patients were considered, the occurrence of clinically relevant increases in serum BUN, which consider the magnitude of the increase in relation to the baseline value, was statistically significantly different between the CKD group and the non-CKD group (p = 0.028; ). The incidence of clinically relevant increases in serum creatinine (p = 0.003; ) and in both serum BUN and creatinine concurrently (p = 0.014, ) was statistically significantly different between the groups. However, there was no significant difference in the occurrence of clinically relevant increases in serum phosphorus between the groups (p = 0.50; ).
Table 5
Clinically relevant serum BUN, creatinine, and phosphorus increase in the CKD group/nonCKD group.
| CKD Group(n = 20) | NonCKD Group(n = 17) | ||
|---|---|---|---|
| Variables | No. of Dogs (%) | No. of Dogs (%) | p-Value |
| BUN elevation | 9 (45%) | 2 (11.76%) | 0.028 |
| Phosphorus elevation | 2 (10%) | 0 (0%) | 0.50 |
| Creatinine elevation | 8 (40%) | 0 (0%) | 0.003 |
| BUN and creatinine elevation | 6 (30%) | 0 (0%) | 0.014 |
4. Discussion
In human patients with impaired renal function, levetiracetam (LEV) dosing is individualized depending on the status of the patients renal function [7,9,12,13], but in veterinary medicine, there have been no reports on individualized LEV dosing according to the status of the patients renal function to date, and there have been few studies on the use of LEV in particular dog populations [19,20]. Thus, the impact of oral LEV on dogs with pre-existing CKD has not been previously reported; the present study provides insights into this matter.
The initial LEV dose was 21.94 8.36 mg/kg (range, 10.045.0 mg/kg) PO, q812h in the CKD group, and 20.58 8.72 mg/kg (range 10.030.0 mg/kg) PO, q812h, in the non-CKD group in this study. In humans, the elimination half-life of LEV is extended in patients with renal impairment [7,8,9,12,13], and LEV dosing is therefore adjusted according to the status of the patients renal function [8,9,12]. Pharmacokinetic studies suggest a dose of LEV 20 mg/kg, q8h to obtain plasma concentrations in normal dogs, which is similar to clinically significant plasma concentrations in humans [21,22]. Consequently, these studies suggest that dogs with renal impairments should be given a LEV dose < 20 mg/kg, q8h [21,22]. It is estimated that some of our CKD patients had higher LEV concentrations than the therapeutic range, as they were administered LEV at doses that were more than 20 mg/kg, q8h [21,22]. We did not obtain serum concentrations of LEV. However, based on previous studies, we assumed that LEV serum concentrations might be higher in the CKD group than in the non-CKD group, which was also concordant with our other study results. Statistically significantly higher number of dogs in the CKD group were reported to have side-effects than those in the non-CKD group (85% vs. 52.94%, p = 0.033). Further study is necessary to determine individualized LEV dosing according to the status of a dogs renal function.
In comparison to other AEDs, LEV has a broad margin of safety [7,8]. In this study, LEV was used as add-on therapy or primary therapy. Many patients in the current study had concurrent diseases because they were middle-aged or older. Because of the minimal hepatic metabolism, LEV is favored for geriatric or critical human patients [7,8,9]. Furthermore, in human medicine, LEV has been shown to have minimal effects on the distribution of other AEDs due to its unique metabolic pathway [7,8,9]. Therefore, the use of LEV in specific complex medical situations, particularly in polytherapy, has been encouraged [10]. In this study, we found that LEV was mostly well tolerated in dogs, but it was not free from side-effects.
In general, the side-effects of LEV include ataxia, sedation, anorexia, polydipsia, vomiting, diarrhea, hypersalivation, and behavior changes (aggression) [10,11,18]. In this study, most patients showed more than one side-effect simultaneously. In the CKD group, there was a significant increase in sedation during LEV administration compared to at baseline, but this increase was not remarkably different from the one observed in the non-CKD group (p = 0.40). Because of sedation, chronic recurrent dehydration associated with periodic water intake may occur [23], which can be problematic, particularly in CKD patients. A key management factor in CKD patients is access to fresh water anytime because the ability to concentrate urine and excrete water is impaired in CKD patients [24]. If a patient with CKD is sedated, water intake may be irregular, which may accelerate dehydration [23], decreasing blood flow to the kidneys [24]. Consequently, uremic toxins are retained [24]. This was supported by the results of the present study. In the CKD group, nine patients (45%) demonstrated clinically significant increases [15,16] in serum BUN () and eight demonstrated clinically relevant increases in serum creatinine (). Moreover, in that group, six patients (30%) had concurrent clinically significant increases [15,16] in serum BUN and creatinine (). On the other hand, in the non-CKD group, there were no clinically significant increases in BUN and/or creatinine (). Therefore, canine CKD patients who are administered LEV should be monitored, and they may require physical and/or laboratory evaluation for dehydration [25,26].
The statically significant difference in the incidence of clinically relevant increases in serum BUN and/or creatinine between the CKD and non-CKD groups () may be due to the acute kidney injury (AKI) that is superimposed on pre-existing CKD. In human medicine, although it has been shown that kidney function can fully recover after overcoming an AKI, cumulative observational data have revealed that AKI can worsen underlying CKD [27]. There have been four case reports in humans where LEV was identified as a possible contributor to AKI [25,26,28,29]. Based on those reports, patients taking LEV who present with abdominal pain, oliguria, nausea/vomiting, and a high creatinine concentration may require laboratory assessment for hidden renal adverse effects [25,26], and renal function during treatment with LEV should be monitored [25,26,28,29]. In the differential diagnosis for any unexplained acute renal failure, AKI due to LEV should be considered [28]. However, in veterinary medicine, there have been no reports of renal toxicity attributed to LEV, and further studies are necessary to assess renal toxicity that is secondary to LEV in dogs.
As in other retrospective studies, this study had some limitations. First, we had some missing values, such as serum SDMA values, which increase during CKD progression and that correlate strongly with a decrease in the glomerular filtration rate [30,31], because the data were collected retrospectively and SDMA has only been included in the diagnostic criteria for the diagnosis of CKD since 2015 [32]. We rarely measured SDMA values before this time-point. Therefore, future studies including SDMA results are needed. Second, in this study, the patients were chronically ill because they were middle-aged or older. Consequently, it is possible that the CKD stage might have been falsely high or falsely low [17,24]; the CKD stage of the patients with prerenal azotemia could have been falsely high [17,24], and that of patients who had lost muscle mass could have been falsely low [17,24]. In retrospective studies, it is difficult to solve this issue. Third, we did not measure the plasma LEV concentration values, as there is no therapeutically effective LEV concentration known in dogs [10]. Therefore, in practice, LEV concentration monitoring is rarely performed [10]. However, based on previous studies, it could be assumed that the CKD group had higher LEV concentrations than the non-CKD group did [7,8,9,21,22]. Lastly, our data could not exclude the possibility that other AEDs (such as phenobarbital, potassium bromide, zonisamide, or diazepam) contributed to anti-epileptic effects or adverse effects. It was also difficult to control the dosage of LEV, owner compliance, and factors in the living environment, such as diet. Further prospective and controlled studies are needed.