acute kidney failure dogs prognosis

Kidney Disease in Dogs: Signs, Symptoms, and Treatment
Your dogs kidneys are essential organs that filter waste products from the bloodstream. When the kidneys are weakened, either by acute or chronic kidney disease, your dogs health could suffer. Because kidney disease progresses over time, its important to learn the common symptoms so tha you can recognize them. If you catch kidney disease in dogs early on, treatment can slow down the progression and allow your dog to live longer.
What Is Kidney Disease in Dogs?
Kidney disease in dogs is sometimes called renal or kidney insufficiency because it occurs when a dogs kidneys stop doing their job as efficiently as they should. The main job of the kidneys is to help clear and excrete waste products from the blood and convert them to urine, says Dr. Jerry Klein, Chief Veterinary Officer for the AKC. If the kidneys are not working properly, these waste products can build up in the blood, causing detrimental effects.
Dogs can get either acute kidney disease, which develops suddenly, or chronic kidney disease (CKD), which develops slowly and worsens over an extended period. Both involve loss of kidney function, but they result from different circumstances. Acute kidney disease is a sudden attack or injury to the kidney, whereas chronic kidney disease is a slow, degenerative loss of kidney function, Dr. Klein explains.
What Causes Kidney Disease in Dogs?
Dr. Klein warns that kidney disease could be caused by a lot of things, including infection (such as with the bacteria that causes leptospirosis), trauma, genetics, drugs, toxins, cancer, mechanical obstructions (like kidney stones), and degenerative diseases (where the job and form of the affected body part get worse over time). Anything that decreases blood flow to the kidneys, such as dehydration or heatstroke, can cause the kidneys to fail.
Acute kidney disease in dogs can be caused by exposure to hazardous materials, including toxic plants such as lilies, certain drugs, harmful foods such as grapes or raisins, or antifreeze. Puppy-proofing your home and yard can keep your dog away from potentially harmful items or foods that could be toxic.
Chronic kidney disease in dogs is also associated with growing older. Because kidney tissue cant regenerate once its damaged, the kidneys can wear out over time. As small-breed dogs often live longer than large-breed dogs, they tend to show early signs of kidney disease at an older age10 years old or more, compared to as young as 7 for the large breeds.
What Are the Symptoms of Kidney Disease in Dogs?
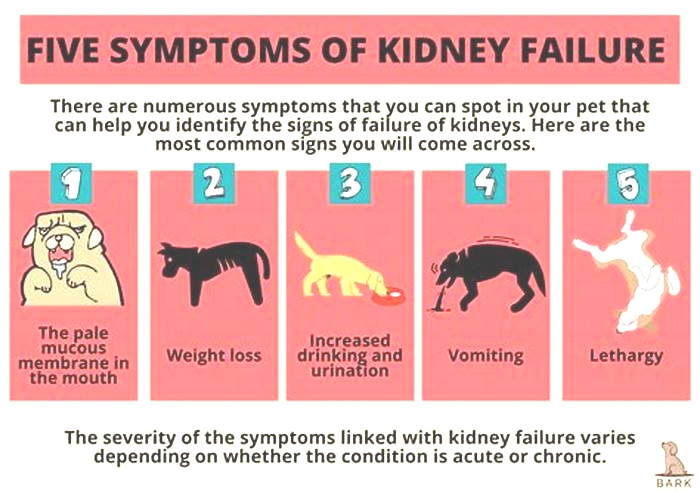
The earliest signs of kidney disease in dogs are increased urination and therefore increased thirst. Other symptoms dont usually become apparent until about two-thirds of the kidney tissue is destroyed. So, in the case of CKD, the damage may have begun months or even years before the owner notices. Because of this, its common for the signs of kidney disease in dogs to seem like they came out of the blue when in fact, the kidneys have been struggling for a long time.
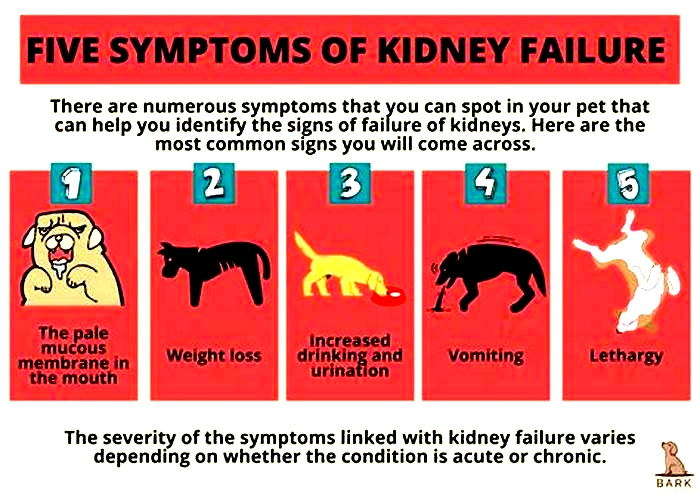
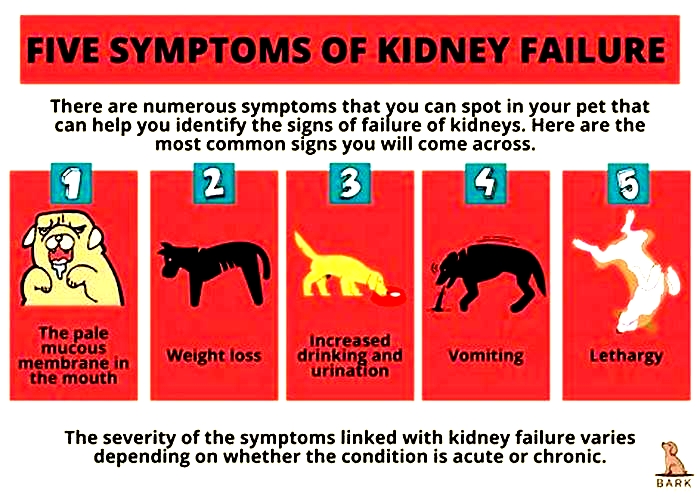
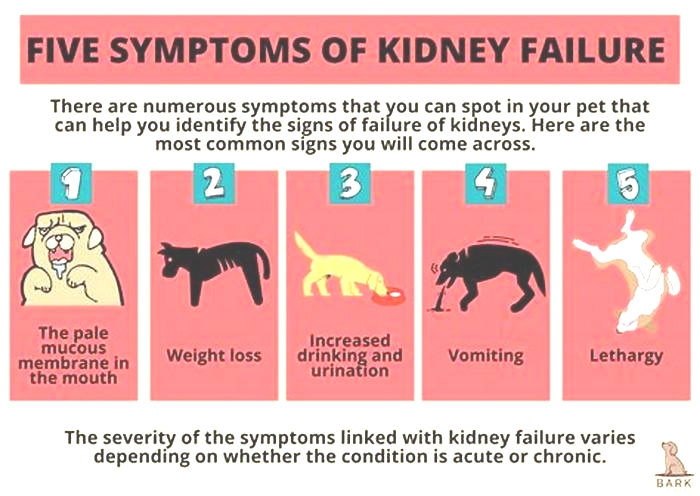
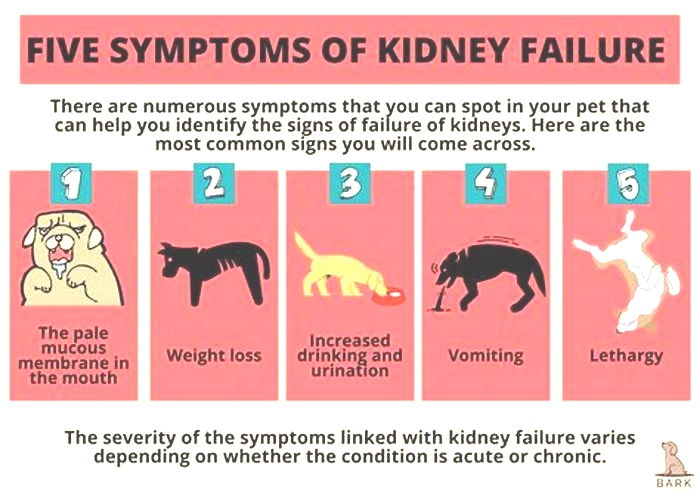
Other signs of chronic kidney disease in dogs to watch for include:
Dr. Klein says there are some rarer symptoms of kidney disease in dogs to be aware of, as well. On occasion, there can be abdominal painurinary obstructions or stonesand in certain instances, one can see ulcers in the oral or gastric cavity. In extreme cases, little or no urine is produced at all.
What Are the Stages of Chronic Kidney Disease in Dogs?
Kidney disease in dogs is measured in stages. Many veterinarians use the IRIS scale, which has four stages. Blood work measurements like creatinine and SDMA (biomarkers for kidney function) allow your vet to assign your dog to a particular stage which will determine the exact treatment.
Dr. Klein explains, The stages determine how well the kidneys can filter waste and extra fluid from the blood. As the stages go up, the kidney function worsens. In the early stages of CKD, the kidneys are still able to filter out waste from the blood. In the latter stages, the kidneys must work harder to filter the blood and in late stages may stop working altogether.
How Is Kidney Disease in Dogs Treated?
Dialysis (a medical procedure that removes waste products and extra fluid from the blood) is far more common in humans than in dogs, although peritoneal (kidney) dialysis can be performed in some cases. On rare occasions, surgical kidney transplant is possible in dogs.
But Dr. Klein specifies that depending on the type and stage of kidney disease, the main treatments for CKD are diet changes and administration of fluids, either directly into the veins (intravenous) or under the skin (subcutaneous). The balancing and correction of electrolytes are extremely important in the management of kidney patients, he explains.
Proper nutrition is needed, and there are many available diets formulated for cats and dogs with kidney issues, some by prescription only. Your veterinarian can help guide you to the most appropriate diet for your pet.
Because kidney disease, particularly in the late stages, can cause a dog to lose their appetite, it can be difficult to encourage your dog to eat enough. Dr. Klein advises, There are medications used as appetite stimulators available, such as the prescription drug mirtazapine. Capromorelin has recently been FDA-approved for dogs to address appetite in chronic kidney disease.
When Do You Need to Call Your Vet?
The prognosis and expected life span for a dog with kidney disease depend on the type of disease, the speed of progression, and underlying conditions present in the dog. However, the more serious the disease, the poorer the outcome. Thats why its so crucial to catch the illness early on.
According to Dr. Klein, In chronic kidney disease, there are methods, such as diets and medications, that can be used to lessen the burden of work the kidneys need to do and may help slow down the progression from one stage to the next. In acute kidney disease, there is less time and fewer choices available to prevent further damage to the kidneys and to try to jump-start the kidneys to get them to function normally.
Regular veterinary exams, including bloodwork, are an excellent way to spot kidney problems before the outward symptoms become apparent. And if you notice any of the above signs, dont hesitate to get your dog to the vet for further testing. It can make a huge difference in preserving kidney function and your dogs well-being for as long as possible.
Kidney Failure in Dogs
What Is Kidney Failure in Dogs?
The primary job of the kidneys is to filter the blood by removing waste products and controlling the amount of fluid and nutrients kept in the body and how much is passed in urine.
With any type of kidney failure, this filtering isnt working well, so waste products are not properly removed from the bloodstream and too much fluid is passed in urine along with proteins and electrolytes. As waste products build up in the blood and tissues, dogs can get ulcerations (tears) in the lining of their digestive tract as well.
Kidney failure may also be referred to by terms listed below. The word renal refers to all things related to kidneys, and is often used interchangeably. Failure, insufficiency, and disease are commonly used to describe similar issues with the kidneys.
Kidney disease is often divided into categories based on how long it has been affecting the dog. Acute renal failure occurs in a very short time frame, and is often caused by eating or drinking a toxin or getting a severe infection that harms the kidneys. Chronic kidney disease refers to a process with a more gradual onset or one that has been happening for a longer period of time.
Changes that can occur with an aging pet are often caused by chronic kidney disease, but if a dogs kidneys were damaged by eating a toxic item several months ago and he now has renal failure because of this, it is also known as chronic kidney disease.
Symptoms of Kidney Failure in Dogs
Drinking more water (polydipsia)
More frequent urination (polyuria)
Urinary accidents in house-trained pets
Lack of energy
Refusing to eat
Vomiting
Drooling
Changes in defecation (either diarrhea or constipation)
Weight loss
Mouth sores
Bad breath
Weakness
Causes of Kidney Failure in Dogs
Kidney failure can occur because of an acute event, such as a toxin ingestion or infection that harms the kidneys; degenerative (worsening) changes over time; or an underlying medical condition that damages renal tissues, which can occur due to genetic predispositions in some dog breeds.
Specific causes include:
Ingested toxins
Metabolic diseases
Kidney infections
Autoimmune disease
Cancers
Breeds that are prone to inherited renal failure include:
How Veterinarians Diagnose Kidney Failure in Dogs
Your veterinarian will want to run several tests, in addition to a physical exam, to diagnose kidney failure, such as:
Complete blood count
Chemistry panel
Urinalysis with culture
Abdominal ultrasound
Treatment of Kidney Failure in Dogs
Treatment of kidney failure is based on the severity of the disease and whether it is acute or chronic.
Acute kidney disease is treated with hospitalization and IV fluid therapy to support the kidneys and help them remove wastes. Depending on the cause of the disease, decontamination medications, toxin-binding medications, antibiotics, or medications to support the gastrointestinal tract may be given. In extreme cases, renal dialysis can help the kidneys. This last procedure is rare, only available at some university or veterinary specialty hospitals.
Chronic kidney disease requires careful management of dogs at home. They need to have access to water at all times and be encouraged to drink water. Many dogs have improvements with a prescription kidney diet. Some dogs need to be on medications to control high blood pressure or to protect their stomach. Pets with chronic kidney disease need to see their veterinarian often so that their renal values can be checked. Some dogs with kidney disease need to receive injectable fluids at home or may even need to be hospitalized at times to help their fluid needs.
Recovery and Management of Kidney Failure in Dogs
With acute kidney failure, prognosis is variable depending upon the cause of the disease, how severe the disease is, how damaged the kidneys are, the speed and aggressiveness of treatment, and the dogs response.
For chronic renal failure, long-term prognosis is not good. Most dogs die or are euthanized within a year because of poor quality of life.
The families of dogs with kidney disease should expect to watch them closely and will need to see their veterinarian often, especially as their pets kidney function gets worse. These dogs will be easily dehydrated, as their kidneys are not able to keep water in their bodies. Any infection, vomiting, diarrhea, or changes in appetite or activity could severely dehydrate the pet and worsen the disease.
Kidney Failure in Dogs FAQs
How does kidney failure differ from kidney disease?
Kidney disease is a broader term that includes any problem with the kidneys. Kidney failure is a specific term that means the kidneys cant keep up with filtering waste products and managing fluid levels.
Is kidney failure fatal in dogs?
Depending on the severity and progression of the disease, kidney failure can be fatal.
WRITTEN BY
Laura Russell, DVM, MBA, DABVPVeterinarian
Dr. Russell is a 2003 graduate of the University of Missouri. She is board certified in Canine and Feline Practice, certified in canine...
Longterm outcome of dogs recovering from acute kidney injury: 132 cases
1.INTRODUCTION
Acute kidney injury (AKI) is characterized by sudden onset of renal parenchymal injury, and often is associated with decreased renal function, retention of uremic waste products, as well as fluid, electrolyte, and acidbase imbalances.1 Disruption of homeostasis and accumulation of uremic toxins are associated with damage to various body organs. Consequently, morbidity and mortality rates remain high despite intensive treatment.2, 3 Shortterm prognosis of AKI is affected by multiple factors including the etiology (which influences the reversibility of the injury), comorbidities, complications, and treatment options.3, 4 In 2 large scale studies of AKI in dogs managed medically or by hemodialysis, shortterm mortality rates were 56% (56/99 dogs) and 47% (86/182 dogs), respectively.2, 3 In the former study, 19/43 dogs (44%) that were discharged from the hospital had serum creatinine concentration (sCr) within the reference range during the followup period, whereas in the remaining 56% sCr remained above the reference range, and subsequently these dogs were diagnosed with chronic kidney disease (CKD). The longterm outcome however was not evaluated in this study.
Other veterinary studies have been focused mainly on a single etiology, and only shortterm outcome has been reported. In a study evaluating the longterm outcome of dogs managed by intermittent hemodialysis, 49/93 dogs (53%) were discharged, and the overall 1year survival was 33% (31/93 dogs).5 Furthermore, this study found that the median overall and renalrelated survival times were significantly longer for dogs with underlying infectious etiology compared to other etiologies.
Information regarding the longterm prognosis of AKI is important for both clinicians and owners. Such data would aid clinicians in decision making and guiding owners' expectations, because AKI is associated with high treatment costs and euthanasia might be considered. Moreover, these data will assist clinicians in tailoring longterm monitoring regimens and possible therapeutic interventions for dogs recovering from AKI. Our objectives were to determine the longterm outcome of dogs recovering from AKI, assess the proportion of dogs with normalization of sCr at discharge and during the followup period, and identify prognostic factors for both normalization of sCr and longterm outcome.
2.MATERIALS AND METHODS
2.1. Case selection
Medical records of dogs presented to the Koret School of Veterinary Medicine, The Robert H. Smith Faculty of Agriculture, Food and Environment, Hebrew University of Jerusalem, diagnosed with AKI and that survived to discharge, between the years 20152021, were reviewed retrospectively. Acute kidney injury was diagnosed based on the International Renal Interest Society (IRIS) guidelines, and included presence of acute onset of clinical signs consistent with AKI (eg, anuria, oliguria, polyuria, vomiting, inappetence) and acute onset of azotemia after exclusion of postrenal causes.1, 6 The maximal documented sCr during hospitalization was used to classify dogs according to IRIS AKI grades. Dogs with a previous diagnosis of CKD or ultrasonographic evidence consistent with CKD (eg, small irregular kidneys, decreased corticomedullary distinction7) were excluded, as were dogs with postrenal azotemia.
2.2. Medical records
Data extracted from the medical records included signalment, history, physical examination findings, CBC, serum biochemistry, urinalysis, urine culture, blood gas analysis, blood pressure measurements, ultrasonographic findings, and hospitalization duration. Dogs were excluded from analysis if they did not survive to discharge or died within 30days after discharge.
The etiology of AKI was classified as follows: (1) inflammatory/ischemic (ie, systemic underlying inflammatory process with suspected decreased tissue perfusion), (2) infectious, (3) nephrotoxic, (4) other, or (5) unknown. Pancreatitis was included in the inflammatory/ischemic category and was diagnosed based on compatible history, clinical signs, ultrasonographic findings, increased serum 1,2odilaurylracglycero glutaric acid(60methylresorufin) ester (DGGR)lipase activity (reference interval, <108U/L) or some combination these.8, 9 Nephrotoxicity was diagnosed based on a history of recent (<72hours before presentation) exposure to a nephrotoxin. Infectious causes included pyelonephritis and leptospirosis. Pyelonephritis was diagnosed based on urine sediment findings (ie, pyuria and bacteriuria), positive urine culture and ultrasonographic findings.10, 11 Leptospirosis was diagnosed if the titer of a single microscopic agglutination test (MAT) was 1:800 without recent (<12months) history of vaccination, or when seroconversion in paired MAT titers (ie, 4fold increase between the first and second samples) was documented.12 Other etiology included AKI secondary to hypercalcemia or glomerulopathies, diagnosed based on the presence of marked proteinuria (urine proteintocreatinine ratio [UPC] >2) after exclusion of extrarenal causes. Hospitalacquired AKI was diagnosed based on an increase in sCr of 0.3mg/dL within 48hours during hospitalization while receiving fluids IV.13 If 2 etiologies were present, the primary cause was designated as the etiology for statistical analysis.
2.3. Laboratory evaluation
Blood samples were collected in potassiumEDTA tubes for CBC (Advia 120 or 2120, Siemens, Erlangen, Germany; Abacus Junior Vet, Diatron, Wien, Austria), and whole blood samples in plain tubes with gel separators for serum biochemistry (Cobas 6000, Roche, Mannheim, Germany). Samples obtained inhouse were analyzed within 60minutes after collection. The CBC and serum biochemistry results from referring clinics were considered if obtained 24hours before admission or during the followup period. Cystocentesis was used to collect urine samples for dipstick chemistry (Urilux, Roche, Mannheim Germany), measurement of specific gravity by refractometry, sediment evaluation, which was done either by experienced laboratory personal or automatically (SediVue Dx, IDEXX Laboratories, Westbrook, ME), and bacterial culture. Pyuria and hematuria were defined as presence of >5 WBCs or RBCs, respectively, per highpower field (hpf). Proteinuria was defined as a urine dipstick result of 1+ (ie, 30mg/dL). The UPC (Cobas Integra 400 Plus or Cobas 6000, Roche, Mannheim, Germany) only was measured in dogs with severe proteinuria (urine dipstick result +4), inactive sediment, and when glomerular disease was suspected as the inciting cause for AKI.
2.4. Followup
Dogs discharged from the hospital but not surviving 30days postdischarge were excluded from the study and the minimum followup time was 30days. Followup data were obtained from the electronic medical records or by telephone interview with the owner or primary care veterinarian or both, and included followup date, status of the dog (ie, alive, dead, euthanized), sCr, and whether the dog had another episode of AKI during the followup period.
Longterm survival of dogs was calculated as the number of days from discharge until the last followup date, death, or euthanasia. Dogs were diagnosed and classified by CKD stage based on the IRIS CKD staging system only if sCr measurements were available >3months after discharge and only if sCr was stable (<10% change between at least 2 measurements >1month apart). Normalization of sCr was defined as sCr <1.4mg/dL at discharge or during the followup period. Dogs eligible for CKD staging in which normalization of sCr was documented were classified as CKD Stage 1.
2.5. Statistical analysis
Continuous variables (eg, age, blood pressure, kidney function variables) were assessed for normality using the ShapiroWilk test. Continuous variables are presented as median and range and categorical variables as proportions. Because many variables were not normally distributed, the MannWhitney U test was used to compare continuous variables between the 2 groups. Chisquared or Fisher's exact tests were used to examine the association between 2 categorical variables. KaplanMeier analysis was performed to assess longterm outcome, and the logrank test was used to determine the effect of categorical variables (eg, pancreatitis, etiology, acute on chronic kidney disease [ACKD], AKI grade) on outcome. Dogs alive at last followup were right censored. Cox regression was used to assess the association of continuous variables (eg, age, blood pressure, kidney function test results) at presentation and maximal documented sCr during hospitalization) with both sCr normalization and longterm outcome. Cox regression also was used to perform multivariable analysis and further assess the relationship between variables found to be associated (P<.1) with longterm outcome in the univariate analysis. All tests were 2tailed, and in all, P<.05 was considered significant. Analyses were performed using a statistical software package (SPSS 22.0 for Windows, IBM Corp., Armonk, N.Y., USA).
3.RESULTS
3.1. Animals and etiology
Medical records search yielded 249 dogs with AKI during the study period, of which 85 dogs did not survive to discharge or 30days postdischarge, and 32 dogs were lost to followup. Thus, 132 dogs met the inclusion criteria and were included, of which 63 were males (36 castrated, 57%) and 69 were females (54 spayed, 78%). The most common breeds were mixed (61 dogs; 46%), German shepherd (9 dogs, 7%), Cavalier King Charles spaniel and Yorkshire terrier (5 dogs each, 4%), Siberian Husky, Labrador retriever, and Maltese (4 dogs each, 3%). Median age was 72months (range, 1216months), and median weight at presentation was 19.3kg (range, 1.275.8kg).
Etiologies were ischemic/inflammatory (70 dogs, 53%) including pancreatitis (21 dogs, 16%), postanesthesia (14 dogs, 11%), severe gastroenteritis (8 dogs, 6%), peritonitis (6 dogs, 4%), heat stroke and severe bleeding (5 dogs, 4% each), diuretic treatment for heart failure (3 dogs, 2%), snake envenomation and myositis (2 dogs, 2% each), pyometra, cycad poisoning, road trauma and cardiac tamponade (1 dog each, 1% each); infectious (15 dogs, 12%), including leptospirosis (14 dogs, 11%) and pyelonephritis (1 dog, 1%); nephrotoxicosis (7 dogs, 5%), including nonsteroidal antiinflammatory drug (NSAID) overdose (6 dogs, 5%), and grape or raisin ingestion (1 dog, 1%). Other etiologies (4 dogs, 3%) included hypercalcemia (3 dogs, 2%) and glomerulopathies (1 dog, 1%). The etiology of AKI was unknown for the remaining dogs (36 dogs, 27%). Hospitalacquired AKI was diagnosed in 13 (10%) dogs.
3.2. Normalization of sCr and laboratory findings during hospitalization
Median sCr at presentation was 3.8mg/dL (range, 1.137.9mg/dL), increasing to 4.1mg/dL (range, 1.143.2mg/dL) during hospitalization. Maximal measured sCr during hospitalization was used to classify dogs by IRIS grades as follows: Grade I, 5 dogs (4%); Grade II, 25 dogs (19%); Grade III, 47 dogs (36%); Grade IV, 35 dogs (26%); and Grade V, 20 dogs (15%). Twentyseven dogs were managed using hemodialysis. Median sCr at discharge was 1.3mg/dL (range, 0.46.7mg/dL). The sCr had normalized in 72 dogs (55%) by the time of discharge. Sixty dogs (45%) were discharged with sCr >1.4mg/dL, of which 27 dogs (45%) had normalization of sCr during the followup period, documented between 2 and 1072days after discharge. Overall, sCr normalized in 99 dogs (75%), either at discharge or during the followup period, of which 76 dogs (76/99, 77%) were alive at the time of last contact.
The sCr at presentation was significantly (P<.001) lower in dogs that experienced sCr normalization at any time point during the followup period (median, 3.3mg/dL; range, 1.115.9mg/dL) compared to dogs without normalization of sCr (median, 7.2mg/dL; range, 1.537.9mg/dL). Similarly, maximal sCr during hospitalization was lower in the former group (median, 3.7mg/dL; range, 1.118.3mg/dL) compared with the latter (median, 7.2mg/dL; range, 2.143.2mg/dL; P<.001; Figure).
Maximal sCr during hospitalization in dogs with and without normalization of sCr. The box represents the second and third quartiles. The horizontal line within the box represents the median. The whiskers represent the range, and the circles indicate outliers. sCr, serum creatinine concentration
The proportion of dogs with sCr normalization (either at discharge or during the followup period) decreased significantly with increase in IRIS AKI Grade (P=.001; Figure). The proportion of dogs with sCr normalization was lower in dogs managed by hemodialysis (27 dogs) compared with dogs managed medically (59% vs 84%, respectively; P=.05). Serum creatinine concentration normalized in 47%, 100%, 81%, 50%, and 72% of dogs with infectious, nephrotoxic, ischemic/inflammatory, other, and unknown etiology, respectively, with significant differences in the proportion of sCr normalization among AKI etiologies (P=.02).
Association between International Renal Interest Society AKI Grade and sCr normalization at both discharge and during the followup period. AKI, acute kidney injury. sCr, serum creatinine concentration
3.3. Longterm outcome
One hundred dogs (76%) were alive at last followup with an estimated median survival time (MST) of 1322days (95% confidence interval [CI], 11471626days; Figure). The proportion of dogs lost to followup after discharge was 10% (13/132) at 3months, 11% (14/132) at 6months, 12% (16/132) at 12months, 32% (42/132) at 2years, and 35% (47/132) >24months after discharge. Thirtytwo (24%) dogs died during the followup period, of which 16 dogs (50%) died naturally, whereas 15 dogs (47%) were euthanized (in 1 dog data regarding the cause of death were missing). Of the 32 dogs that died during the followup period, 12 died because of nonurinary related causes (hemoabdomen, cardiac tamponade, neoplasia, and post seizure), 10 dogs died due to further progression of their kidney disease, and in 10 dogs the cause of death could not be verified. After discharge, the proportion of surviving dogs was 95% (125/132 dogs) at 3months, 90% (118/132 dogs) at 6months, 87% (115/132 dogs) at 12months, and 81% (107/132) at 24months. The remaining 5% (7/132 dogs) died >24months after discharge. A significant association was found between age and longterm outcome (P<.001; hazard ratio, 1.015; 95% CI, 1.0081.022).
Long term followup in dogs surviving acute kidney injury
Clinical signs at presentation, hypertension, and pancreatitis were not associated with sCr normalization or longterm survival. No significant association was found between longterm survival and IRIS AKI grade (P=.06). No difference was found in longterm survival between dogs managed medically or using hemodialysis (P=.15). No association was identified between longterm survival and normalization of sCr at any time (P=.63), but significant differences in longterm survival were found among the AKI etiologies (P=.004; Figure); none of the dogs with infectious etiology died during the followup period.
Association between etiology of acute kidney injury and longterm survival. AKI, acute kidney injury
Dogs in which sCr did not normalize during followup period had median sCr concentration of 1.9mg/dL (range, 1.46.5mg/dL) at last followup. In 32 dogs, sCr was not stable during the followup period, hence these dogs were not classified by CKD Stage, and 14 of these dogs experienced a continuous decrease in sCr up to 3months after discharge. Sixtyfour dogs had stable sCr 3months after discharge and were classified by IRIS CKD stages as follows: 49/64 dogs (77%) were classified as IRIS CKD stage 1 and 15/64 dogs (23%) were classified as IRIS CKD stage 2.
Sixtyfive dogs with sCr normalization at any time had sufficient followup data (ie, 1 sCr after sCr normalization) to assess whether azotemia recurred, and azotemia was documented in 9 of these dogs (14%), 2191077days after discharge.
3.4. AKI during the followup period
Fourteen dogs (11%) developed another episode of AKI (ie, ACKD) during the followup period, within a median time of 222days (range, 41309days). These dogs were significantly (P=.01) older (124months; range, 10174months) compared to dogs that did not develop ACKD (median, 72months; range, 1216months). The etiologies of ACKD were ischemic/inflammatory (9 dogs, 64%) including pancreatitis (4 dogs, 29%) and diuretic treatment for heart disease (2 dogs, 14%), acute hepatitis (1 dog, 7%), severe gastroenteritis (1 dog, 7%), and immunemediated hemolytic anemia (1 dog, 7%). The etiology for ACKD was unknown in 5 (36%) dogs. No difference was found in the proportion of dogs that developed ACKD between dogs with or without normalization of sCr at any time (10% vs 12%; P=.75). Longterm survival of these dogs was significantly shorter (P<.001; hazard ratio, 3.7; 95% CI, 1.87.8) compared to dogs that did not develop ACKD (median, 677days; 95% CI, 397957days vs median, 1549days; 95% CI, 13221778days, respectively).
3.5. Multivariable analysis
In a multivariable Cox regression model including age, ACKD, etiology and AKI Grade, only age (P<.001) and etiology (P=.02) remained statistically significant whereas ACKD did not (P=.07).
4.DISCUSSION
Dogs that recovered from AKI and survived >30days had a good longterm prognosis in our study. Normalization of sCr was documented in 75% of the dogs, either at discharge or during the followup period.
Acute kidney injury is a severe syndrome, often requiring prolonged and costly hospitalization, with overall mortality as high as 45% to 62%, even with advanced treatment.2, 3, 14, 15, 16 Despite its acute nature and potential for reversibility, AKI also may affect longterm outcome, because not all surviving dogs regain completely normal kidney function. The latter sustain CKD and as such, are prone to progression, because CKD in dogs is progressive in nature, and thus is expected to influence longterm survival. This information likely influences decision making of both clinicians and owners once AKI has been diagnosed. Currently, large scale studies describing the longterm outcome of dogs recovering from AKI are lacking.
In our study, the estimated MST of dogs recovering from AKI was 1322days (95% CI, 11471626days), longer than previously described in veterinary studies.1, 2, 55 This rather long survival time is probably an underestimation of the actual MST, because many of the dogs were still alive at last followup. In accordance with previous studies, sCr normalized in approximately half of the animals by the time of discharge.2, 17 However, our results further documented that sCr normalization occurs in many dogs after discharge. Indeed, sCr normalized during the followup period in 45% of the dogs discharged with sCr above the reference range, and subsequently sCr normalized in 75% of the dogs, which is a higher proportion than previously described (44%).2
As might be expected, the proportion of dogs with sCr normalization decreased significantly with increase in IRIS AKI grade (Figure). Yet, longterm survival was not associated with IRIS AKI Grade. The magnitude of azotemia during AKI represents the severity of kidney dysfunction, but not necessarily the potential for reversibility, which is highly influenced by the underlying cause.18 The role of etiology in AKI recovery and shortterm outcome has been well established in several studies,3, 5 and also is documented here by differences in the proporations of dogs with sCr normalization among different AKI etiologies. In our study, the effect of etiology on longterm outcome also is demonstrated. Etiology affects the nature of the injury and the availability of specific treatments to eliminate the underlying cause. It is likely that some etiologies exert their effect also after recovery from the acute insult (e.g., glomerulopathies) whereas others do not (e.g., infectious).
The time until sCr normalization during the followup period varied, and could not have been assessed very accurately because normalization occurred between 2 succeeding visits. Because of the nature of our study, the time interval between visits was not constant and was sometimes long. In some dogs, sCr normalized within a few days after discharge, whereas in others normalization was documented after several months. These results suggest that renal recovery and repair processes after AKI may last weeks to months before a new steady state is reached.
During AKI recovery, 2 separate processes may occur: renal repair and activation of compensatory mechanisms in the remaining nephrons (ie, compensatory hypertrophy). Renal repair processes include repopulation of the tubules by regenerating epithelial cells that also undergo maturation.19, 20 Under certain circumstances, these repair mechanisms may lead to maladaptive renal changes, resulting in persistent renal dysfunction and CKD.19, 21 Simultaneously, compensatory hypertrophy and hyperfiltration occur, as the surviving nephrons compensate for the loss in renal functional mass.22, 23 Compensation processes might result in a gradual decrease in sCr over months after the abrupt impairment in kidney function.24 The term AKD (acute kidney disease) has been proposed to represent the time window after AKI during which renal pathophysiologic processes may be active and ongoing.19, 25, 26, 27 The prolonged recovery phase documented in our study is consistent with guidelines used in humans, indicating that CKD can only be diagnosed after AKI when azotemia persists and is stable for >3months.28 This guideline also should be considered in veterinary medicine, because the time for sCr normalization in our cohort was as long as 3months. This new information regarding the extended recovery process should be taken into consideration before making final diagnoses and clinical decisions.
Despite the high proportion of dogs with resolved azotemia, it is plausible that irreversible parenchymal damage and decreased renal function are present in dogs with apparent clinical recovery and normalization of biochemical variables. These changes might not be reflected in traditional renal functional markers (eg, sCr and symmetric dimethylarginine), and thus a diagnosis of CKD stage 1 should be made in all dogs recovering from AKI. Dogs with CKD are at increased risk for developing uremic crises (ie, ACKD),29 thus recognition of stage 1 CKD, including routine monitoring, is important. Indeed, the proportion of dogs developing another episode of AKI was not different between dogs that had normalization of sCr and dogs that did not. Increased owner awareness about mild clinical signs is important, because it also has been shown that ACKD carries a guarded longterm prognosis in both dogs and cats.30, 31 Early diagnosis of ACKD will facilitate therapeutic intervention and might improve outcome. The fact that 14% of the dogs with sCr normalization had recurrence of azotemia further supports the notion that despite apparent complete recovery from AKI, dogs with sCr normalization after an AKI episode should be considered as stage 1 CKD (or at least to have higher risk for CKD and ACKD), and thus should be monitored and treated appropriately. Dogs diagnosed with another episode of AKI during the followup period in our study were significantly older compared to dogs that did not, and therefore older dogs recovering from AKI may need closer monitoring.
Dogs without normalization of sCr >3months after the acute insult were considered to have azotemic CKD (stage 2 or higher). The MST of these dogs is apparently longer than is reported for dogs with CKD, and likely results from a slow CKD progression rate in dogs recovering from AKI.32, 33 The reason for this apparently slower progression might be related to the degree of compensatory hypertrophy and the processes governing progression in CKD. It is possible that, once substantial kidney function is regained (as occurred in our study), compensatory maladaptive processes that occur in further progression of CKD23 do not occur to their full extent, or do not occur at all. Indeed, in our study, azotemic dogs eligible for CKD staging only were classified as IRIS CKD stage 1 or 2. Another potential explanation for the apparently slow progression may be related to CKD etiology. The etiology of CKD often is unknown at the time of diagnosis, but likely exerts its negative effect on kidney function throughout the disease course, contributing to further progression of the disease. These factors might not be present in dogs with CKD after AKI, and therefore would not promote progression, and thus, in the absence of compensatory hypertrophy and activation of maladaptive processes, kidney function may remain relatively stable. Another plausible explanation for the lack of difference in survival time between dogs with or without normalization of sCr relates to the fact that half of the dogs discharged with sCr above the reference range showed a decrease in sCr during the followup period. Thus, sCr normalization potentially also had occurred in these dogs, but was never documented. The notion of slow CKD progression after AKI also is supported by the fact that 12/22 dogs (55%) with a known cause of death during the followup period died of causes unrelated to their kidney disease, and only 10 dogs died or were euthanized because of CKD progression.
Our study had some limitations, mostly related to its retrospective design. Incomplete medical records might have weakened the power of some statistical analyses. Classification of some etiologies could not be proven and these therefore were presumed. It is possible the severity of azotemia at presentation was affected by dehydration in some of the dogs, but because evaluation of hydration status is very subjective and was assessed by multiple clinicians during the study period, we elected not to include this information. The followup period varied among dogs, because some were discharged only months before final analysis, resulting in a relatively short followup period, and other were still alive years after discharge, and censored, possibly underestimating MST. After discharge, monitoring and treatment of dogs were performed by different clinicians, at primary care clinics, using different machines (with potentially different reference ranges), which possibly affected our results. We did not use breedspecific reference ranges to define sCr normalization although a sCr <1.4mg/dL could be considered high for some (especially miniature) breeds, thereby overestimating sCr normalization. Relevant kidney function variables (eg, blood urea nitrogen concentration, urine specific gravity) were not evaluated consistently during the followup period and thus could not be included in the overall assessment of kidney function. Differentiation between renal and nonrenal related death during the followup period was not always possible. Because most of the dogs had normalization of sCr and some of the other dogs were not eligible for CKD staging, the number of dogs that were included in the CKD staging was relatively low.
In conclusion, our findings suggest that dogs recovering from AKI have a relatively good longterm outcome. In most of the dogs, sCr normalized at discharge, and a substantial number of dogs experienced sCr normalization within the following weeks to months after discharge. Normalization of sCr was less likely to occur with increasing IRIS AKI grade, but normalization was not associated with longterm survival, suggesting slow progression rate of CKD after AKI. Significant differences in sCr normalization and longterm survival were observed among AKI etiologies, emphasizing the importance of reversibility of renal injury rather than the severity of renal dysfunction during AKI to the longterm outcome. These findings should facilitate clinical decision making and provide owner guidance during hospitalization as well as dictate longterm monitoring of dogs with AKI.